Product Name: HSP27
Product Number: AB-NN152-3
| Size: | 50 µg | Price: | 89.00 | |
| $US |
Target Full Name: Heat shock protein beta-1
Target Alias: 28kDa heat shock protein; CMT2F; HSP25; HSP27; HSP28; HSPB1; SRP27
Product Type Specific: Heat shock/stress protein pan-specific antibody
Antibody Code: NN152-3
Antibody Target Type: Pan-specific
Protein UniProt: P04792
Protein SigNET: P04792
Antibody Type: Monoclonal
Antibody Host Species: Mouse
Antibody Ig Isotype Clone: IgG2b Kappa
Antibody Immunogen Source: Human HSP27
Production Method: Protein G purified
Target Alias: 28kDa heat shock protein; CMT2F; HSP25; HSP27; HSP28; HSPB1; SRP27
Product Type Specific: Heat shock/stress protein pan-specific antibody
Antibody Code: NN152-3
Antibody Target Type: Pan-specific
Protein UniProt: P04792
Protein SigNET: P04792
Antibody Type: Monoclonal
Antibody Host Species: Mouse
Antibody Ig Isotype Clone: IgG2b Kappa
Antibody Immunogen Source: Human HSP27
Production Method: Protein G purified
Antibody Modification: Unconjugated. Contact KInexus if you are interest in having the antibody biotinylated or coupled with fluorescent dyes.
Antibody Concentration: 1 mg/ml
Storage Buffer: Phosphate buffered saline pH7.4, 50% glycerol, 0.09% sodium azide
Storage Conditions: For long term storage, keep frozen at -40°C or lower. Stock solution can be kept at +4°C for more than 3 months. Avoid repeated freeze-thaw cycles.
Product Use: Western blotting | Immunohistochemistry | ICC/Immunofluorescence | Immunoprecipitation | ELISA
Antibody Dilution Recommended: WB (1:2000), ICC/IF (1:100); optimal dilutions for assays should be determined by the user.
Antibody Concentration: 1 mg/ml
Storage Buffer: Phosphate buffered saline pH7.4, 50% glycerol, 0.09% sodium azide
Storage Conditions: For long term storage, keep frozen at -40°C or lower. Stock solution can be kept at +4°C for more than 3 months. Avoid repeated freeze-thaw cycles.
Product Use: Western blotting | Immunohistochemistry | ICC/Immunofluorescence | Immunoprecipitation | ELISA
Antibody Dilution Recommended: WB (1:2000), ICC/IF (1:100); optimal dilutions for assays should be determined by the user.
Antibody Potency: Very high potency. Detects a ~27 kDa protein in cell and tissue lysates by Western blotting. Very limited cross-reactivity to other non-human species.
Antibody Species Reactivity: Human
Antibody Positive Control: 0.5 µg/ml of SMC-161 was sufficient for detection of HSP27 in 10 µg of HeLa lysate by colorimetric immunoblot analysis using Goat anti-mouse IgG:HRP as the secondary antibody.
Antibody Specificity: Very high
Antibody Cross Reactivity: Has no cross-reactivity to Alpha B crystallin.
Related Product 1: HSP27 pan-specific antibody (Cat. No.: AB-NN152-2)
Antibody Species Reactivity: Human
Antibody Positive Control: 0.5 µg/ml of SMC-161 was sufficient for detection of HSP27 in 10 µg of HeLa lysate by colorimetric immunoblot analysis using Goat anti-mouse IgG:HRP as the secondary antibody.
Antibody Specificity: Very high
Antibody Cross Reactivity: Has no cross-reactivity to Alpha B crystallin.
Related Product 1: HSP27 pan-specific antibody (Cat. No.: AB-NN152-2)
Scientific Background: HSP27s belong to an abundant and ubiquitous family of small heat shock proteins (sHSP). It is an important HSP found in both normal human cells and cancer cells. The basic structure of most sHSPs is a homologous and highly conserved amino acid sequence, with an α-crystallin domain at the C-terminus and the WD/EPF domain at the less conserved N-terminus. This N-terminus is essential for the development of high molecular oligomers (1, 2). HSP27-oligomers consist of stable dimers formed by as many as 8-40 HSP27 protein monomers (3). The oligomerization status is connected with the chaperone activity: aggregates of large oligomers have high chaperone activity, whereas dimers have no chaperone activity (4). HSP27 is localized to the cytoplasm of unstressed cells but can redistribute to the nucleus in response to stress, where it may function to stabilize DNA and/or the nuclear membrane. Other functions include chaperone activity (as mentioned above), thermo tolerance in vivo, inhibition of apoptosis, and signal transduction. Specifically, in vitro, it acts as an ATP-independent chaperone by inhibiting protein aggregation and by stabilizing partially denatured proteins, which ensures refolding of the HSP70 complex. HSP27 is also involved in the apoptotic signaling pathway because it interferes with the activation of cytochrome c/Apaf-1/dATP complex, thereby inhibiting the activation of procaspase-9. It is also hypothesized that HSP27 may serve some role in cross-bridge formation between actin and myosin (5). And finally, HSP27 is also thought to be involved in the process of cell differentiation. The up-regulation of HSP27 correlates with the rate of phosphorylation and with an increase of large oligomers. It is possible that HSP27 may play a crucial role in termination of growth (6). Looking for more information on HSP27? Visit our new HSP27 Scientific Resource Guide at http://www.HSP27.com.
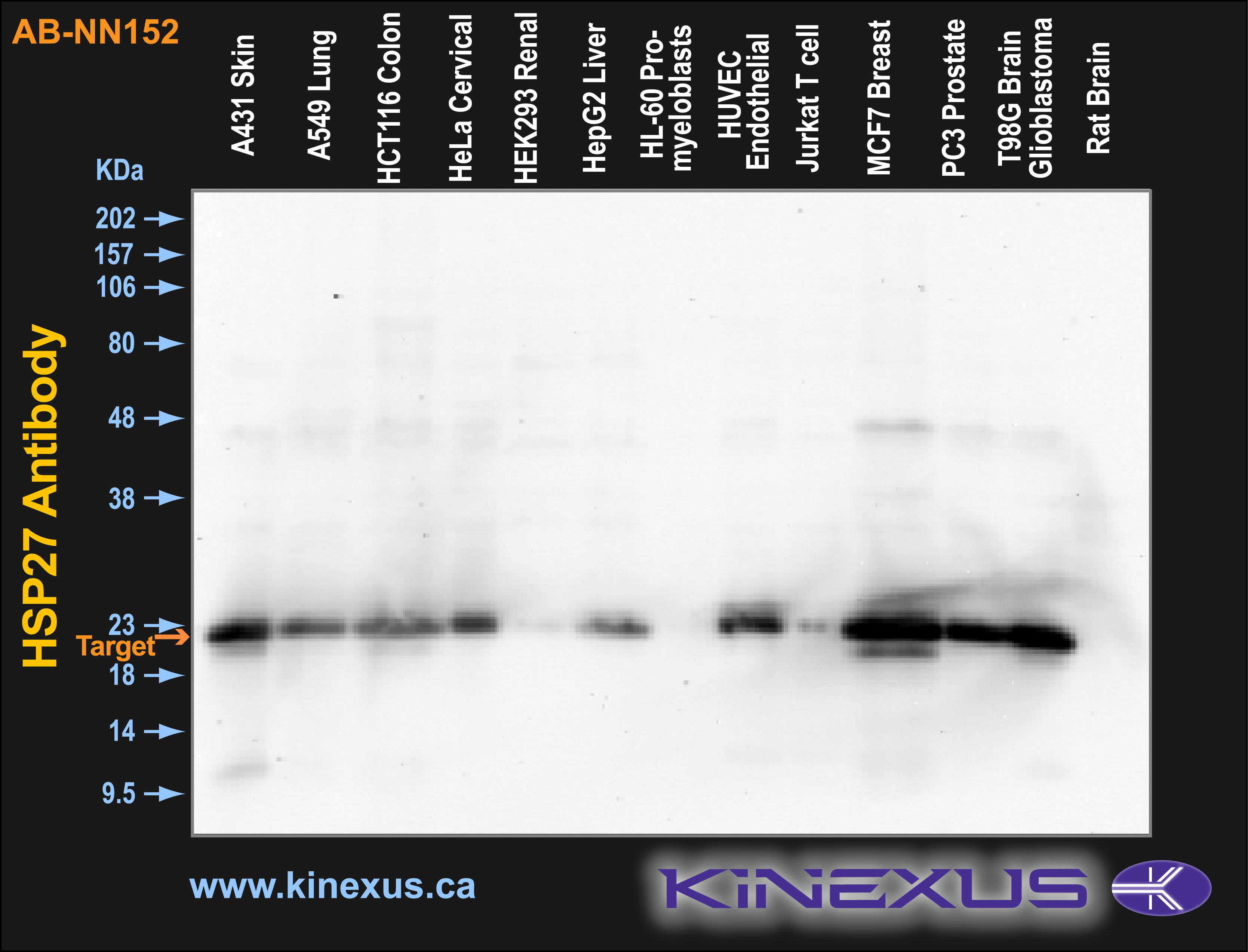
Figure 1. Immunoblotting of various cell lines with AB-NN152 antibody at a 1 µg/mL final concentration. The target protein Hsp27 is indicated. Each lane was loaded with 15 µg of cell lysate protein. The max signal count was 48769.
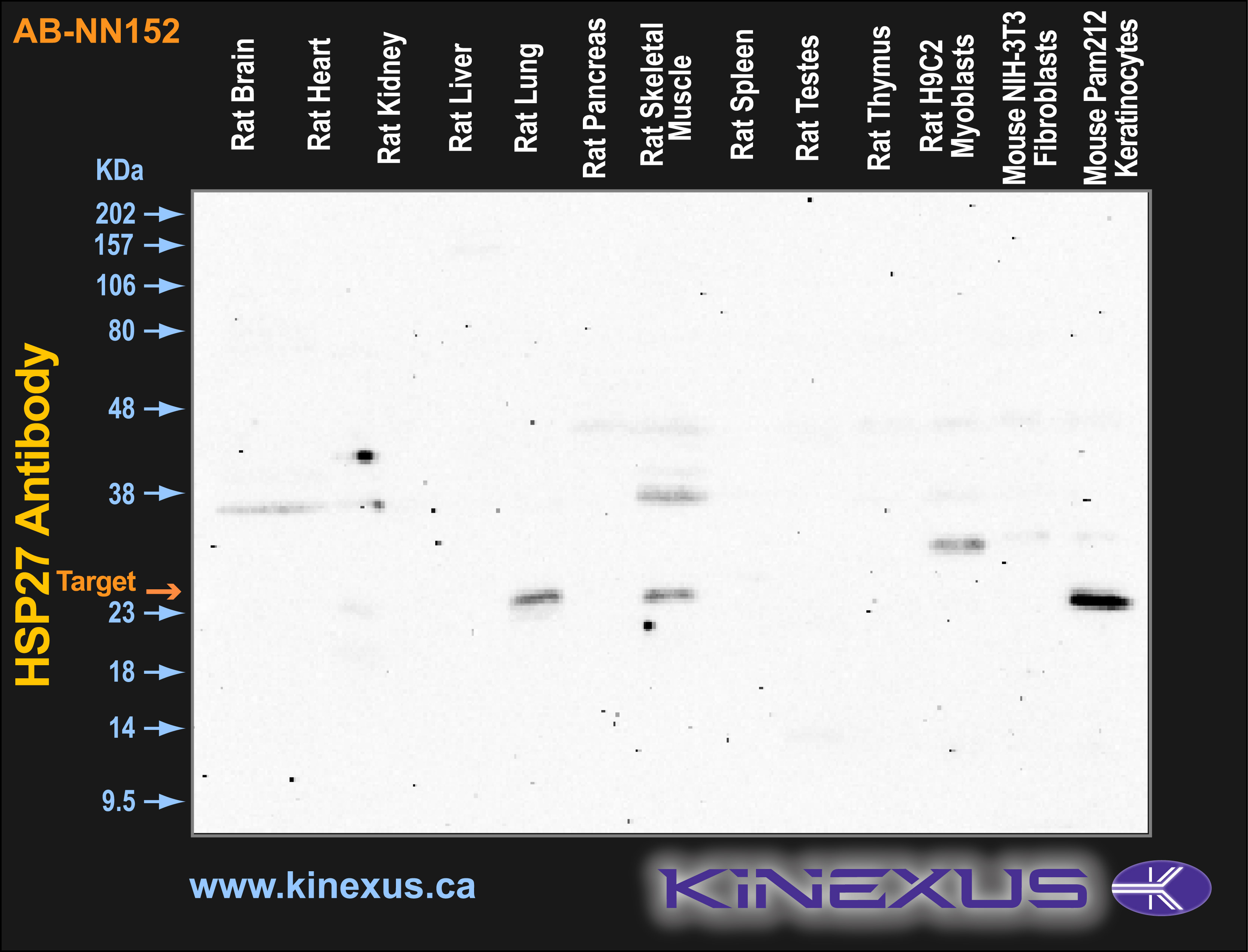
Figure 2. Immunoblotting of various tissue lines with AB-NN152 antibody at a 1 µg/mL final concentration. The target protein Hsp27 is indicated. Each lane was loaded with 15 µg of tissue lysate protein. The max signal count was 5646.
References
[1] Kim K.K., Kim R., and Kim, S. (1998) Nature 394(6693): 595-599.
[2] Van Montfort R., Slingsby C., and Vierling E. (2001) Addv Protein Chem. 59: 105-56.
[3] Ehrnsperger M., Graber S., Gaestel M. and Buchner J. (1997) EMBO J. 16: 221-229.
[4] Ciocca D.R., Oesterreich S., Chamness G.C., McGuire W.L., and Fugua S.A. (1993) J Natl Cancer Inst. 85 (19): 1558-70.
[5] Sarto C., Binnz P.A., and Mocarelli P. (2000) Electrophoresis. 21(6): 1218-26.
[6] Arrigo A.P. (2005) J Cell Biochem. 94(2): 241-6.
[1] Kim K.K., Kim R., and Kim, S. (1998) Nature 394(6693): 595-599.
[2] Van Montfort R., Slingsby C., and Vierling E. (2001) Addv Protein Chem. 59: 105-56.
[3] Ehrnsperger M., Graber S., Gaestel M. and Buchner J. (1997) EMBO J. 16: 221-229.
[4] Ciocca D.R., Oesterreich S., Chamness G.C., McGuire W.L., and Fugua S.A. (1993) J Natl Cancer Inst. 85 (19): 1558-70.
[5] Sarto C., Binnz P.A., and Mocarelli P. (2000) Electrophoresis. 21(6): 1218-26.
[6] Arrigo A.P. (2005) J Cell Biochem. 94(2): 241-6.
© Kinexus Bioinformatics Corporation 2017